Physics Primer: The Photoelectric Experiment
物理入门:光电实验
The PHotoelectric Experiment
光电实验
The photoelectric effect is illustrated bythe following experiment: We shine light on a metal plate, called aphotocathode (or cathode). We use a special light source for which we can varythe frequency. Above a certain frequency, the photocathode will emit electronsthat are collected by a second metal plate. We can connect the two metal platesby a wire and an ammeter to measure the intensity of the current we haveproduced. If we want to determine the kinetic energy of the emitted electronswe can also connect to the circuit a power supply that adds a voltage betweenthe two metal plates.
光电效应是通过以下实验来证明的:我们将光照射在金属板上,称为光电阴极(或阴极)。我们使用一种特殊的光源来改变频率。在一定频率以上,光电阴极将发射由第二金属板收集的电子。我们可以用一根电线和一个电流表连接两块金属板来测量我们产生的电流的强度。如果我们想确定发射电子的动能,我们也可以连接电路,在两个金属板之间增加一个电压。
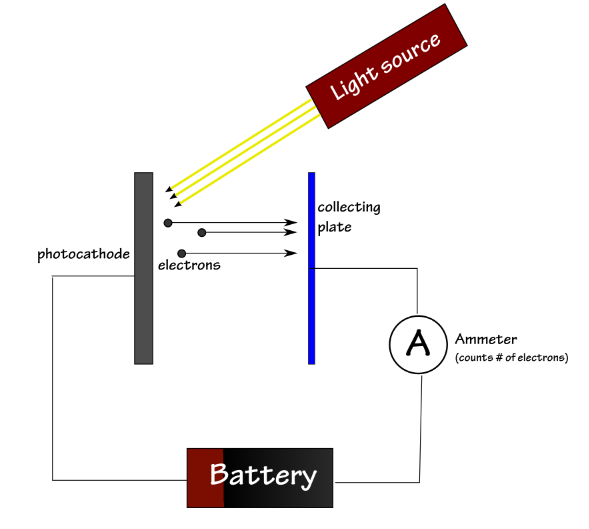
When the electrons are emitted by thephotocathode they have a certain velocity and kinetic energy Ekinetic.When we introduce the voltage (and implicitly the electric field) the electronswill be slowed down. Above a certain voltage, the electrons would stopcompletely between the two metal plates, and they would accelerate back towardsthe photocathode. The minimum value of the voltage for which the electrons turnback corresponds to an electric energy that matches the initial kinetic energyof the electron:
当电子通过他们有一定的速度和动能Ekinetic阴极发出。当我们引入电压(隐含电场)时,电子就会慢下来。在一定电压以上,电子将完全停止在两个金属板之间,它们会加速回到光电阴极。电子折返的电压的最小值对应于与电子的初始动能匹配的电能:
Eelectric=Ekinetic
The electric energy corresponding to thevoltage is: Eelectric=e⋅V,where e is the charge of an electron and V is the voltage.
电压对应的电能:Eelectric=e⋅V,其中e是电子电荷的电压V。
For the purpose of this course it is notnecessary to know where this equation is coming from, but if you are wonderingabout it you should know that this relation defines the electric voltage: avoltage "is the electric energy charge difference of electric potentialenergy transported between two points" (http://en.wikipedia.org/wiki/Voltage)
为了这个课程的目的,没有必要知道这个方程式是从哪里来的,但是如果你想知道它的话,你应该知道这个关系定义了电压:电压是两个点之间传输的势能的电能电荷差”(http://en.wikipedia.org/wiki/Voltage)。
Using this relation we can find the kineticvalue of the emitted electrons:
利用这种关系,我们可以发现发射电子的动能值:
Ekinetic=eV
Using this setup, we can measure and thenplot the energy of the electrons emitted by the cathode versus the frequency ofthe light that is pointed towards the cathode. The result would look like theplot bellow:
利用这个装置,我们可以测量和绘制阴极发射电子的能量与指向阴极的光的频率。结果看起来像故事情节:
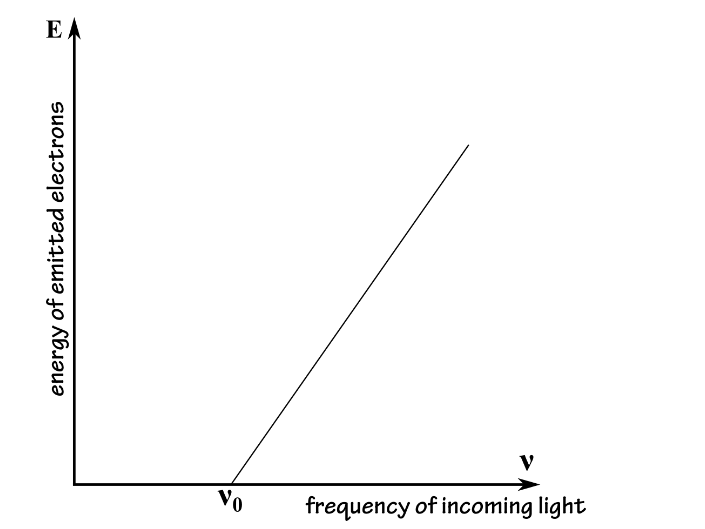
This result could not be explained by theclassical theory of light waves. According to classical physics, the energycarried by a wave of light is proportional to its intensity, so the energytransmitted to the escaping electrons would be expected to vary with theintensity of the incident light: the brighter the light, the more energetic theelectrons. Likewise, there shouldn’t have been any lower threshold frequencyneeded to eject the electrons: a bright enough light should have carried enoughenergy to free the electrons from the metal even for a low frequency. Instead,the energy carried by each electron was independent of the intensity of theincident light rays; only the number of emitted electrons depended on theintensity of light, but not their energy.
这个结果不能用经典的光波理论来解释。根据经典物理学,光波所携带的能量与其强度成正比,所以传送到逃逸电子的能量将随着入射光强度的变化而变化:光线越亮,电子能量就越大。同样地,不应该有任何低阈值的频率来驱逐电子:足够明亮的光应该携带足够的能量来释放电子,即使是低频的金属。相反,每个电子所携带的能量与入射光的强度无关,只有发射电子的数量取决于光的强度,而取决于它们的能量。
Einstein's Solution
爱因斯坦的解决办法
Opposed to the other physicists whounanimously treated light as a wave, Einstein assumed that one could considerlight as being made out of discrete particles (which were later dubbed“photons”). Einstein used these particles as a "heuristic", a usefulbut provisional explanation that did not necessarily correspond to the truenature of light.
与其他一致认为光是波浪的物理学家不同,爱因斯坦认为人们可以认为光是由离散粒子(后来称为光子)制成的。爱因斯坦用这些粒子作为“启发”,一个有用但暂时的解释,并不一定符合光的本质。
How would the photoelectric effect lookfrom the point of view of the atoms in the cathode, if light behaved like aparticle? Before the light source is turned on, the electrons in the cathodeare bound to their atoms with a certain binding energy, which is acharacteristic of the specific kind of metal used in the cathode, and which wemay denote by Φ. In order to be torn free of their host atoms, the electronswould need to receive an amount of energy in excess of this binding energy. (Sofar, both the wave theory and the particle theory of light would agree withthis assumption.)
如果光线表现得像粒子,那么从阴极中的原子的角度来看,光电效应是怎样的呢?在打开光源,在阴极的电子束缚其原子具有一定的结合能,这是金属的特定用于阴极的特性,而我们可以用Φ。为了摆脱它们的宿主原子,电子需要接受超过这个结合能的能量。(到目前为止,波理论和光的粒子理论都会同意这一假设。)
The light source is switched on. Instead ofa continuous wave of light washing over the surface of the cathode, imagine theelectrons in the cathode being hit by lots and lots of separate photons. Eachphoton would carry a definite amount of energy, given by Planck's equation:
光源接通。而不是连续的光在阴极表面冲刷,想象阴极中的电子被大量不同的光子击中。每个光子都有一定量的能量,由普朗克方程给出:
Ephoton=hν
Each time one of the photons hits anelectron in the cathode, it would collide much as billiard balls collide,transferring its energy to the electron. If the energy of the incoming photon wasless than the electron’s binding energy, the electron, even after absorbing thephoton’s energy, would be unable to escape from its atom: no ejected electrons,and no measured current. Maybe counterintuitively, if two photons hit anelectron immediately one after the other, the energy they would transmit to theelectron will not add up. After being hit by a photon, the electron might bemore energetic for a very brief period of time, but almost immediately it willfall back in its low energy state. When the second photon hits the electron,the electron will be back in its initial state.
每当一个光子击中阴极中的一个电子,它就会碰撞,就像台球碰撞一样,把能量传递给电子。如果入射光子的能量小于电子的结合能,即使吸收光子能量后,电子也无法从原子中逸出:没有被逐出的电子,也没有可测量的电流。也许与直觉相反,如果两光子击中电子立即一前一后,他们的能量会传递给电子不会增加。在被光子击中后,电子在一段很短的时间内可能会更有活力,但几乎立刻就会回到低能状态。当第二光子击中电子时,电子将回到初始状态。
Think of a garbage can half-filled withping pong balls. You are trying to get some of the balls out by throwingpennies in. If you throw the pennies with insufficient energy, it doesn’tmatter how many pennies you throw, you won’t bounce a ping-pong ball out. Yetif you throw even a single penny with enough energy, you can bounce a ball outof the can. A photocathode exposed to high-frequency light works in a similarway. If the incoming photons carried more energy than the binding energy, thenafter colliding with an electron and transferring its energy, the electronwould be torn free from its atom, and would stream toward the collecting platewith a kinetic energy
想想一个装满乒乓球的垃圾桶。你想通过掷硬币来获得一些球。如果你把精力不足的硬币扔出去,不管你扔多少便士,你都不会把乒乓球弹出去。然而,如果你投一个硬币,只要你有足够的能量,你就能把球从罐里弹出来。接触高频光的光电阴极的工作方式类似。如果入射光子携带的能量超过束缚能,然后与电子碰撞并传递能量,电子将从原子中被撕裂,并以动能向收集板流去。
Ekinetic=hν−Φ
By thinking of light as made up of theseparticle-like photons, rather than as a continuous wave, Einstein could explainall the features of the photoelectric effect: a single photon (carrying energyEphoton=hν) would be sufficient to knock out an electron, and henceproduce a current in the circuit, so long as hν>Φ. A greater intensity ofthe light source would just mean more photons, but each carrying the sameenergy hν. With more photons hitting the surface, more collisions betweenphotons and electrons would take place, resulting in more electrons beingemitted — yet each electron, having been knocked free by its collision with anincoming photon, would carry the same energy, hν−Φ. Thus, even a very intenselight would be unable to release any electrons (and hence would produce nocurrent) if the energy carried by its photons fell below the binding energy.
通过思考光是由这些粒子如光子,而不是作为一个连续波,爱因斯坦可以解释光电效应的特点:单光子(携带的能量Ephoton = Hν)足以摧毁一个电子,从而产生电流的电路,只要Hν>Φ。较大强度的光源就意味着更多的光子,但都带着同样的能量ν。更多的光子击中表面,光子和电子之间的碰撞会发生,导致更多的电子被发射-但每个电子,已经被与入射的光子碰撞,将携带相同的能量,Hν−Φ。因此,即使一个非常强烈的光将无法释放任何电子(因此不会产生电流),如果其光子携带的能量低于束缚能。