Practice Quiz
练习测验
Simulating the Photoelectric Effect
光电效应模拟
None of the questions below are graded. Thepurpose of the practice quiz is to familiarize you with a simulation of thephotoelectric effect that we'll be using in the graded Quiz. To open thesimulation, click here. Note, this simulation requires Flash. If you using on adevice that cannot support Flash, please skip this exercise.
下面的问题没有一个是评分的。练习测验的目的是让你熟悉我们将在等级测验中使用的光电效应的模拟。要打开模拟,请单击此处。注意,这个模拟需要flash。如果您在不能支持Flash的设备上使用,请跳过此练习。
This simulation allows you to change theexperimental value of four variables:
这个模拟允许你改变四个变量的实验值:
1. From the menu you can select"Options" and "Metals" to change the metal from which theemitting plate (the photocathode) is made. The default value is Sodium. You cansee this in the upper right corner where it says "Selected cathode".The value of the binding energy Φ depends on the type of metal.
1、从菜单中你可以选择“选项”和“金属”来改变发射板(光电阴极)的金属。默认值是钠。你可以在右上角看到“选择阴极”。Φ价值取决于金属的类型。
2. You can change the wavelengthof the incoming light by either moving the upper slider, or by directlyinserting a value in the upper left box. Notice that the upper right boxindicates the value of the corresponding frequency (this value is given by therelation: "speed of light" = "wavelength" x "frequency").If the frequency is not high enough (i.e. the photons' energy is not highenough) then no electrons will be emitted.
2、通过移动上滑块,或者直接在左上角插入一个值,可以改变入射光的波长。注意,右上角框表示相应频率的值(这个值是由关系给出的:“光的速度”=“波长”x“频率”)。如果频率不够高(即光子的能量不够高),那么就不会发出电子。
3. You can change the intensity ofthe light using the lower left slider (if you press on # you can directlyintroduce a numerical value). A higher light intensity corresponds to a higherdensity of photons.
3。你可以改变光强利用左下方的滑块(如果按#可以直接引入一个数值)。较高的光强对应于较高的光子密度。
4. You can change the voltagebetween the plates using the lower right slider (if you press on # you candirectly introduce a numerical value). Any positive voltage between the platescorresponds to an electric force that pulls the electrons back towards the emittingplate (the photocathode). When the electrons are emitted by the photocathodethey have a certain velocity and kinetic energy Ekinetic. When youintroduce a voltage (and implicitly an electric field) the electrons will bedecelerated - you can easily see in the simulation that they are being sloweddown. Above a certain voltage, all the electrons will return back to thephotocathode. The minimum value of the voltage, V0, for which the electronsturn back corresponds to an electric energy that matches the initial kineticenergy of the electron:
4。你可以改变右下滑块的板之间的电压(如果按#可以直接引入一个数值)。板之间的任何正电压对应的电能将电子拉向发射板(光电阴极)。当电子通过他们有一定的速度和动能Ekinetic阴极发出。当你引入一个电压(隐式的电场)电子将减速-你可以很容易地看到在模拟,他们正在放缓。在一定电压下,所有的电子都会返回到光电阴极,V0的电压最小值,其中的电子返回对应的电能与电子的初始动能:
Eelectric=Ekinetic
The electric energy corresponding to thevoltage is: Eelectric=e⋅V,where e is the charge of an electron and V is the voltage.
电压对应的电能:电子= E⋅V,其中e是电子电荷的电压V。
For the purpose of this course it is notnecessary to know where this equation is coming from, but if you are wonderingabout it you should know that this relation defines the electric voltage: avoltage "is the electric energy charge difference of electric potentialenergy transported between two points" (http://en.wikipedia.org/wiki/Voltage).
为了这个课程的目的,没有必要知道这个方程式是从哪里来的,但是如果你想知道它的话,你应该知道这个关系定义了电压:电压是两个点之间传输的势能的电能电荷差”(http:维基百科)。
Using this relation we can find the kineticvalue of the emitted electrons:
利用这种关系,我们可以发现发射电子的动能值:
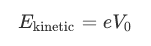
where 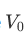 is the minimum voltage.
is the minimum voltage.
 是最小电压。
是最小电压。
Recall from the previous section that thekinetic energy of the emitted electrons corresponds to the energy of theincoming photons, minus some binding energy Φ - this value corresponds to theenergy the electrons need to receive to escape the metal. If you play with thesimulation you will notice that this value depends on the metal from which thephotocathode is made. Then we can also write:
回忆起所发射的电子的动能,对应的光子能量的前一部分,减去一些结合能Φ-这个值对应于电子需要逃避金属能量。如果你玩模拟,你会注意到,这个值取决于金属制成的光电阴极。然后我们也可以写:
Ekinetic=hν−Φ
Thus knowing the minimum voltage for whichthe electrons turn back, we can compute the value of Φ:
因此,了解最低电压,电子转回来,我们可以计算Φ价值:
eV0=hν−Φ
Φ=hν−eV0
There are two ways of spotting the minimumvoltage:
发现最小电压有两种方法:
1. you can follow the behavior ofthe electrons as you increase the voltage. The minimum voltage corresponds tothe case in which the electrons almost reach the collecting plate, stop rightin front of it, and then they turn back.
1、当你增加电压时,你可以跟随电子的行为。最小电压对应于电子几乎到达收集板的情况,在它的正前方停下来,然后再折回。
2. You can follow the indicationsof the Ammeter. When the electrons reach the collector plate, the Ammeter willshow some non-zero value. Watch carefully the Ammeter as you increase the valueof the voltage - its value is going to decrease because fewer electrons reachthe collecting plate. The minimum voltage corresponds to the voltage value forwhich the Ammeter shows for the first time zero.
2、你可以按照安培表的指示。当电子到达收集器板时,电流表会显示一些非零值。仔细观察安培表,当你增加电压的值-它的值会减少,因为更少的电子到达收集板。最小电压对应于电流表第一次显示的电压值。
You can also find Φ by determining thethreshold frequency, ν0, for which electrons are being emitted. This valuecorresponds to the case in which all the energy of the photon is used to freethe electron from the metal, so the kinetic energy of the electrons is zero. Inthis case:
你也可以通过确定阈值频率,发现Φ,ν0,其中电子被发射。这个值对应于光子的所有能量被用来从电子中释放电子的情况,所以电子的动能是零。在这种情况下:
hν0−Φ=0
Φ=hν0
The values of Φ you would obtain using thetwo methods should coincide (up to an approximation factor). If you want tocompare the binding energies of two different metals, it's enough to compareeither their threshold frequencies or their minimum voltages.
价值观的Φ你将获得使用这两种方法应一致(最多一个近似因子)。如果你想比较两种不同金属的结合能,就可以比较它们的阈值频率或者它们的最小电压。